Sterile Anesthesia Medications for Hospitals & Surgical Centers
Anesthesia medications are essential to clinical workflows across operating rooms, surgical centers, emergency departments, and critical-care units.
At OutSourceWoRx, we provide ready-to-use sterile anesthesia preparations manufactured under cGMP, USP <797>/<800>, and rigorous quality-control processes, ensuring accuracy, sterility, and consistency for every batch.
Our goal is simple: support hospital pharmacists, anesthesiologists, and surgical teams with a reliable, compliant, and scalable anesthesia supply.
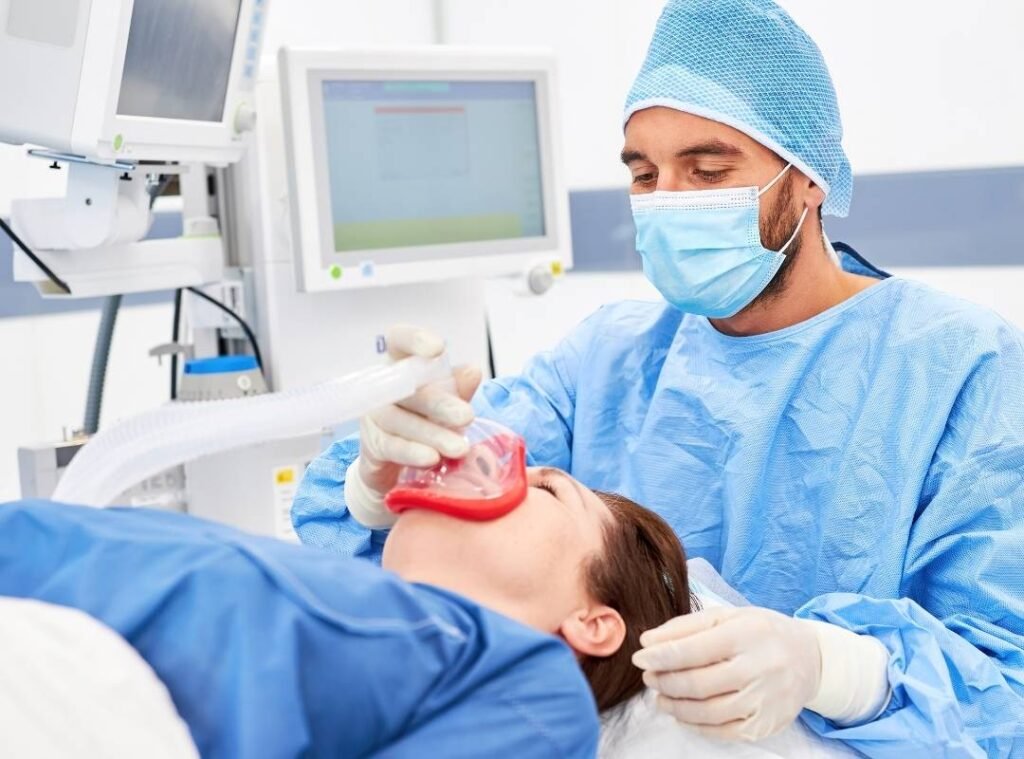
What Are Anesthesia Medications?
Anesthesia medications include a broad range of sterile injectables and related agents used to:
- Induce anesthesia
- Maintain sedation
- Manage pain
- Support airway and respiratory stability
- Facilitate surgical procedures
These medications play a critical role in:
Why Hospitals Outsource Anesthesia Medications to 503B Facilities
Hospital pharmacy teams rely on 503B outsourcing for anesthesia medications due to the high stakes of sterility, consistency, and compliance.
Ensures standardized quality and reliable production.
Each batch undergoes:
- Sterility testing
- Endotoxin testing
- Potency verification
- Environmental monitoring
503B facilities help stabilize availability when medications are in short supply.
Outsourcing helps hospitals reduce compliance risk associated with in-house sterile compounding.
Eliminates the need for hospital teams to compound high-risk sterile injectables internally.
Available upon request for internal compliance reviews.
How OutSourceWoRx Ensures Sterility & Quality
Every anesthesia preparation undergoes our multi-step QC framework:
Our operational model ensures reliability in high-acuity clinical settings, where anesthesia medications must be accurate and immediately ready for use.
Ordering & Workflow Overview
We designed our workflow to support hospitals, surgical teams, and outpatient practices with clarity and efficiency.
Providers submit their request through the portal or by coordinating with our clinical team.
Medications are prepared or released according to their respective batch protocols.
Orders are packaged with strict control measures and delivered to hospitals, ASCs, or clinics.
Our provider relations team supports implementation, documentation requests, and any ongoing needs.
Who We Serve
OutSourceWoRx supports healthcare organizations across:
Benefits of Partnering with OutSourceWoRx
Sterile medications ready when OR and ICU teams need them.
Manufacturing aligned with FDA, cGMP, and USP standards.
Fewer compounding tasks mean more time for clinical priorities.
Validated sterile processes reduce risk for frontline teams.
From routine operations to high-volume procedural days.
Frequently Asked Questions (FAQ)
All medications follow cGMP manufacturing and USP <797>/<800> sterile-compounding standards.
Yes—our preparations support OR, ICU, ED, outpatient surgery, and procedural care.
Through validated sterile processes, QC testing, environmental monitoring, and batch documentation.
Yes. Documentation is available upon request for regulatory and internal reviews.
Our team is available to discuss workflows, onboarding, and operational needs.
Request Provider Information
Our team supports hospitals, ASCs, and medical groups in maintaining safe, reliable anesthesia supply. Connect with us to discuss your needs.
OutSourceWoRx delivers excellence as a 503B compounding pharmacy supplier, providing sterile, compliant medications with innovation, reliability, and clinical expertise.
Main Navigation
Resources & Support
Contact Us
1600 Silver Beach Rd Ste 300 Lake Park FL 33408
+1 561-346-4077
Info@OutSourceWoRx.com
Copyright 2025, OutSourceWoRX. All Rights Reserved.


